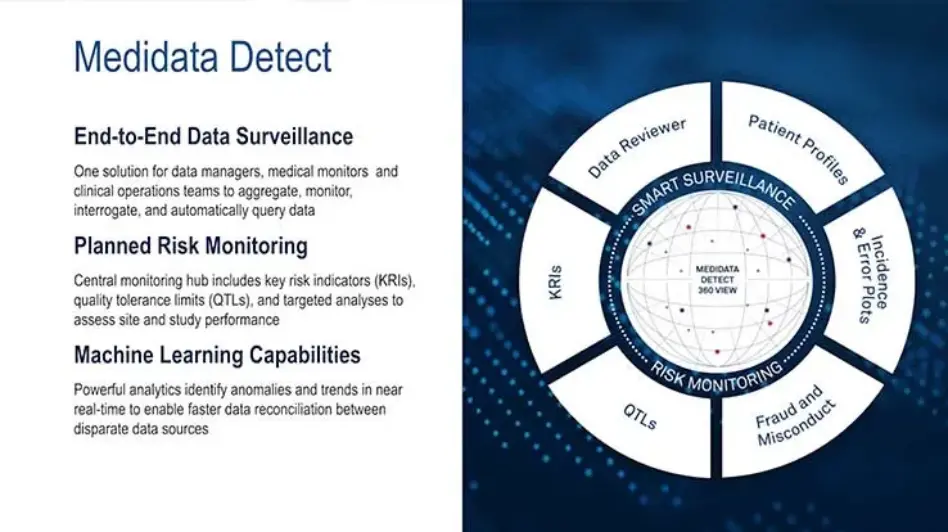
Medidata Detect
Comprehensive Data and Risk Surveillance
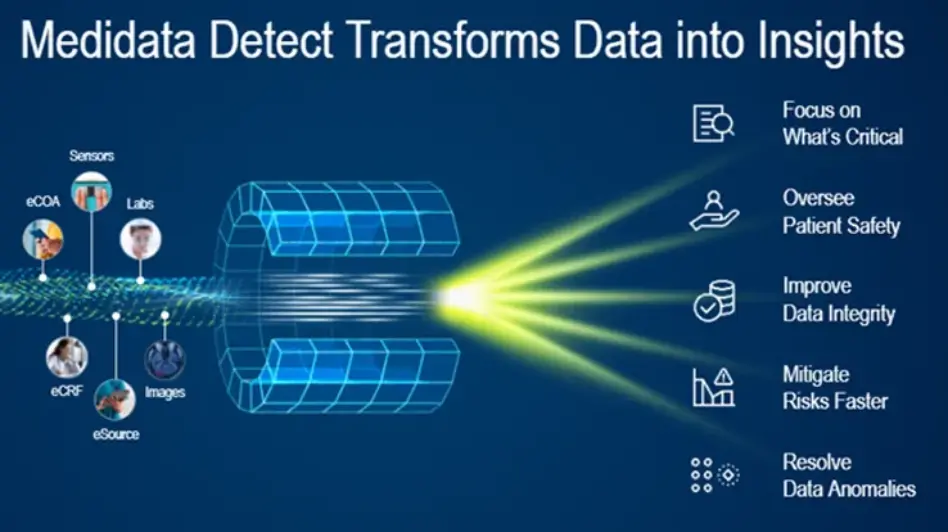
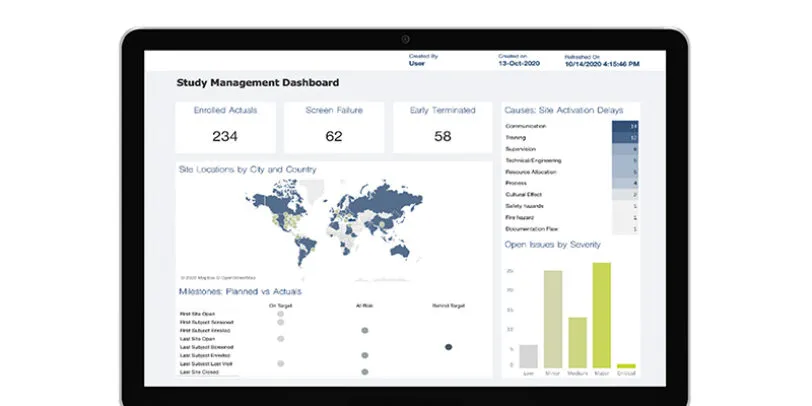
The increased complexity of data collected in today’s trials raise new challenges for managing data quality. Medidata Detect solves these challenges in a comprehensive data surveillance and risk management solution that allows cross-functional teams to monitor and mitigate risks to data integrity and patient safety. Powered by data aggregated from many sources, Detect provides clean, integrated patient data and delivers simplified patient reviews, site performance monitoring, anomaly detection, and faster time to database lock. Detect ensures data quality and improves efficiency with real-time insights delivered in intuitive and actionable readouts.
Why Choose Medidata Detect?
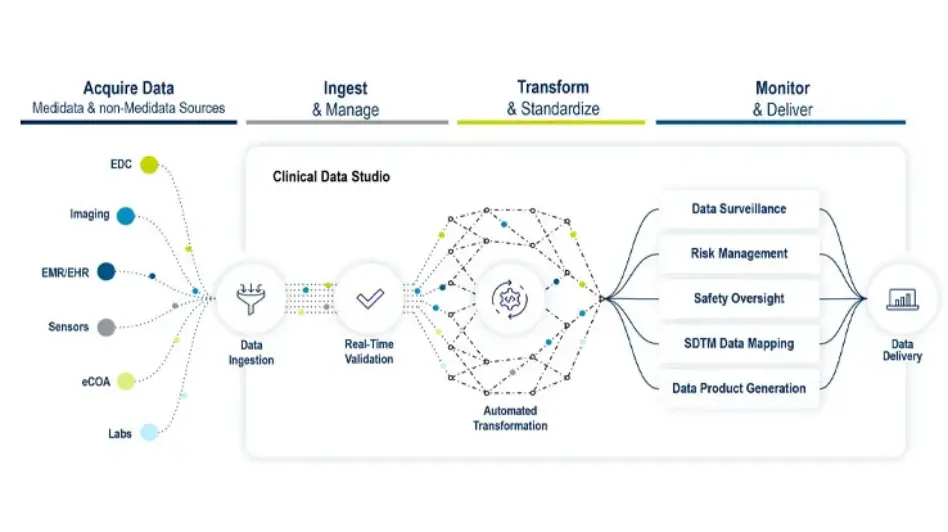
Unlock the True Potential of Your Clinical Trial Data
Detect is part of the new data experience Medidata Clinical Data Studio. Clinical Data Studio is an intuitive, consistent data experience that unlocks the true power of clinical research data. Powered by Medidata’s unified platform and enhanced with AI, Clinical Data Studio streamlines data integration, standardization, management, and usage processes.
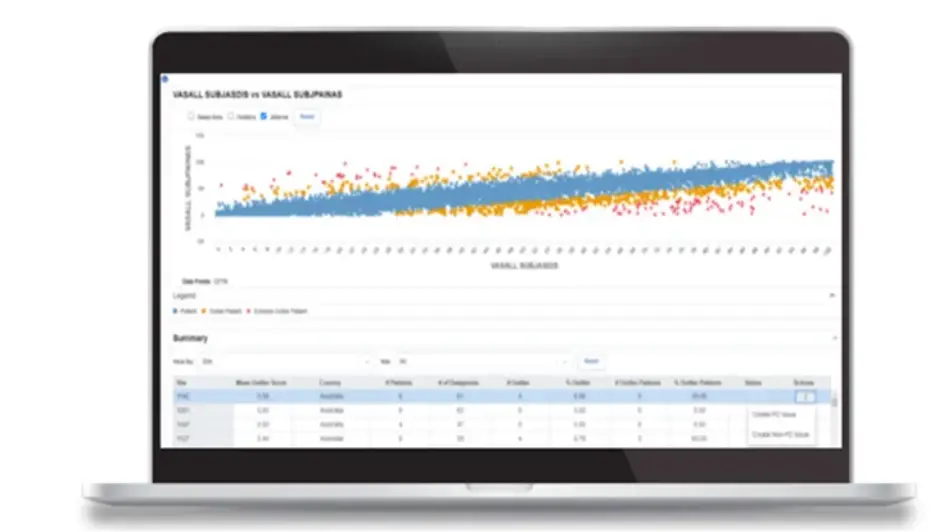
Actionable Insights Powered by Advanced Analytics
Advanced analytics are the foundation of Medidata Detect, including machine learning, AI, and intelligent process automation. Insights are delivered in intuitive and actionable outputs that support multiple roles in a clinical study, regardless of statistical expertise or programming knowledge. Self-service options include the configuration of custom KRIs, QTLs, and listings, filtering on subsets of data within various analytic representations, and creating trial-specific visualizations and analytics.
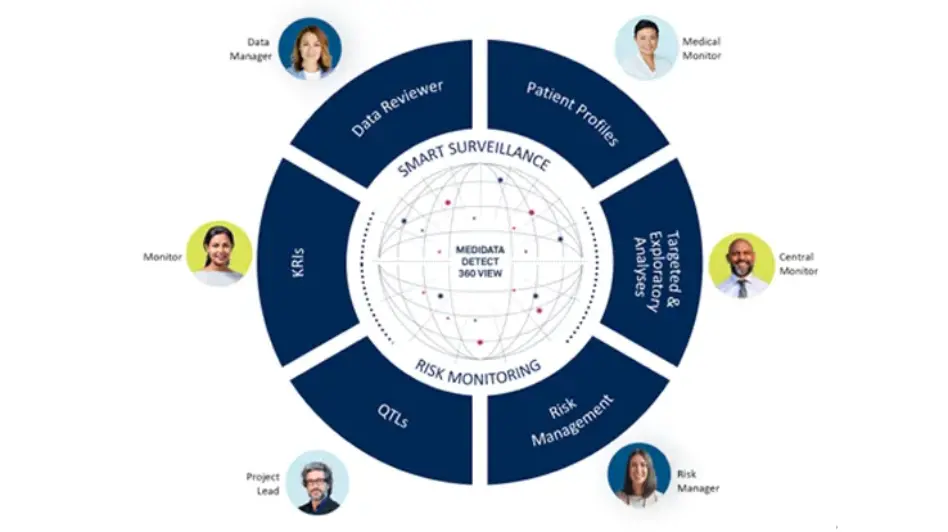
Powerful, Modular Capabilities Deliver Role-Based Activities
Medidata Detect’s capabilities are modularized to enable role-based workflow execution, supporting cross-functional users including data managers, CRAs, central monitors, medical monitors, and clinical project managers. Detect enables multiple stakeholders to manage quality and risk throughout the trial, and is fully integrated with an issue management interface to allow team members to assign issues to CRAs for follow-up during communication with sites.
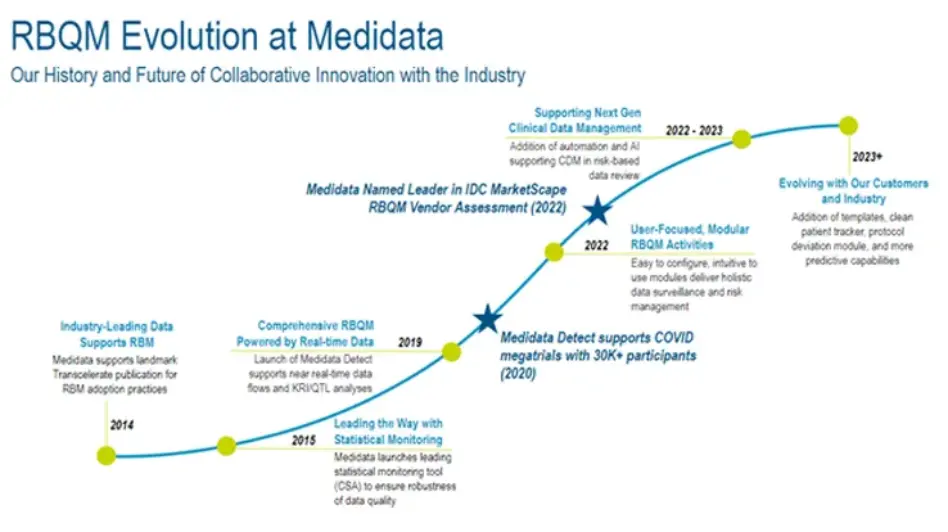
A History of Innovation within the Industry
Medidata Detect was built by experts with extensive direct Risk-based Quality Management (RBQM) operational experience. Medidata was recently named by independent research firm IDC as a leader in the “IDC MarketScape Worldwide Life Science R&D Risk-Based Monitoring Solutions Vendor Assessment for 2022.” This report evaluates capabilities using criteria life science companies should consider when selecting a strategic RBQM solution provider.
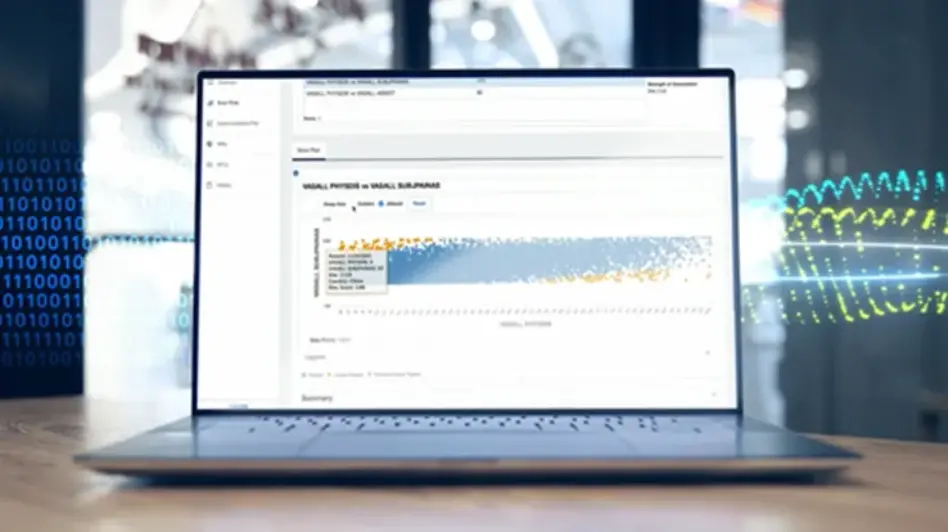
Centralized Monitoring Enhances Compliance and Data Consistency
The increased adoption of decentralized methodologies, including electronic clinical outcome assessments (eCOA) and electronic patient-reported outcomes (ePRO) has created a new challenge: ensuring patients comply with completing questionnaires and diaries. Centralized monitoring supported by Medidata Detect is one of the best approaches to identify potential noncompliance quickly so patients and sites can be contacted by the sponsor or CRO for root cause analysis and retraining.
Key Features of Medidata Detect

Accelerate Data and Patient-Level Review
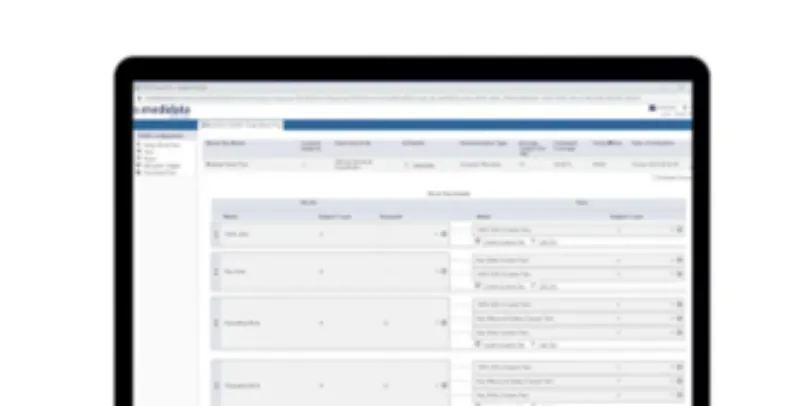
Patient Data Surveillance includes Data Reviewer and Patient Profiles applications. Data Reviewer allows data managers to automate data review and reconciliation with AI, simplify with bulk query management, and track patient cleaning status in real-time dashboards. Patient Profiles enables intuitive, in-depth patient safety reviews across all data sources.

Key Risk Indicators
Key Risk Indicators (KRIs) give monitors and other clinical team members the ability to track site and country performance and compliance.
Medidata Detect provides ten industry-standard KRIs out of the box, and additional custom KRIs can be created in a matter of minutes from Rave EDC or external datasets. The KRIs module is fully integrated with closed-loop issue management.

Quality Tolerance Limits
Medidata Detect supports identifying systemic and protocol-level issues with Quality Tolerance Limits (QTLs). The QTLs module provides robust risk monitoring capabilities via statistical process control and predictive capabilities.

Centralized Statistical Monitoring
Centralized Statistical Monitoring (CSM) provides targeted, easy-to-use analyses for data integrity as well as a machine learning-driven engine for the detection of missing data, data anomalies, gaps, and unusual data association at the data point, patient, site, or country level.

Risk Management
Medidata Risk Management gives sponsors and CROs the visibility and power to manage oversight and risk during study planning through trial conduct. It is an integrated digital solution that identifies critical-to-quality (CtQ) factors, associated risks, and mitigation strategies and monitors for those trial risks through key risk indicators (KRIs) and quality tolerance limits (QTLs).

Comprehensive Data Surveillance Reporting
Remove the stress of the unknown with Detect360 Reporting. RBQM Operational Advisors can provide a complete review of your study data to surface potential observations, present these observations via a report and workshop, and guide issue identification and risk mitigation.
Related Solutions
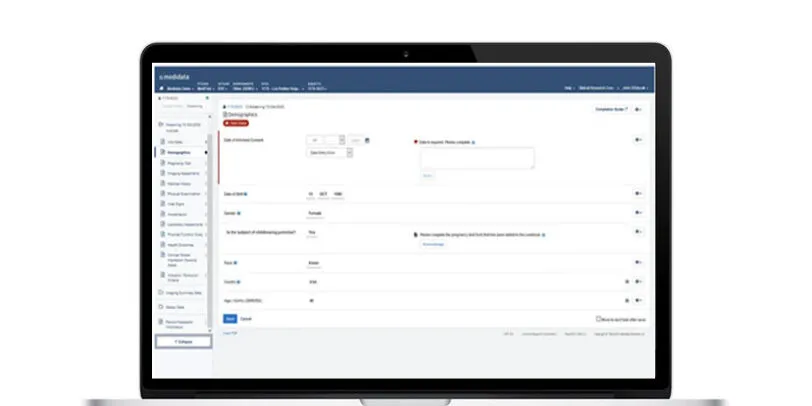
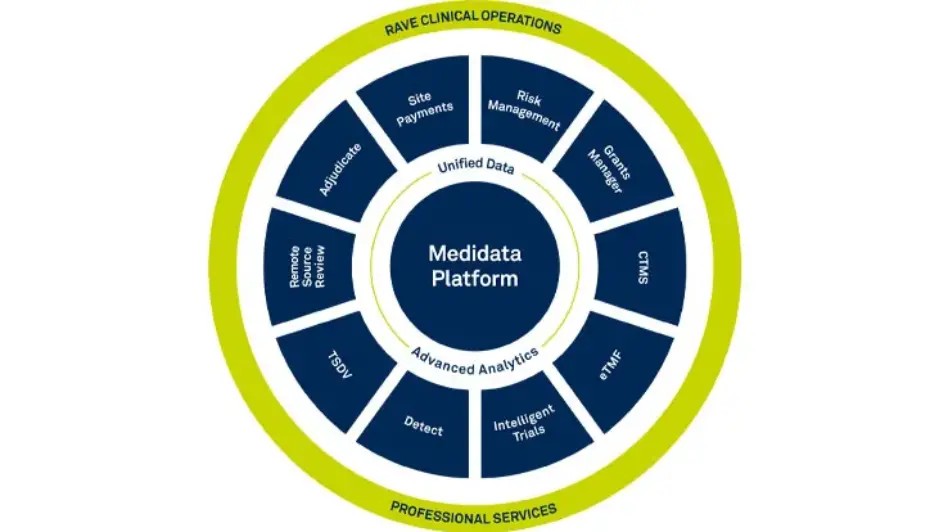
Rave EDC
Medidata’s Rave EDC (Electronic Data Capture) is the most advanced, robust and secure EDC system for all clinical trial data capture and management. Rave EDC is the cornerstone of the Medidata Platform that connects processes, eliminates data reconciliation, and delivers cross-functional and cross-study data insights.
Rave TSDV
Rave TSDV (Targeted SDV) identifies Rave EDC folders, forms, and data fields that will be selectively ‘targeted’ for SDV. It enables your CRAs to efficiently take action on critical to quality (CtQ) factors identified within risk management activities.
Rave CTMS
Rave CTMS is a unified digital solution that improves the speed, efficiency, and collaboration for the oversight of your studies, countries, and sites through automation and workflow management.
Learn More

A (R)evolutionary Path for Data Managers
To stay ahead of the disruptive clinical trial data evolution, learn how technology continues to inform and optimize clinical data strategies and management processes with the “5Vs”—Volume, Variety, Velocity, Veracity, and Value.

Avoiding Risk in Clinical Trials
Organizations are now adopting risk-based quality management (RBQM) strategies to ensure the quality, integrity, and reliability of clinical trial data. Powerful and innovative RBQM solutions can help you mitigate and manage risk holistically across all your trials. Read this blog series on how to avoid risks in your studies.

Improving Clinical Trial Oversight with Central Monitoring
Read this white paper to learn how proper adoption of risk-based quality management strategies, including centralized monitoring, can shorten trial timelines, reduce overall costs, and improve trial outcomes.
Moderna Chooses Medidata Detect to Ensure Data Integrity and Patient Safety
Moderna used Medidata Detect to help them with early risk identification and proactive mitigation planning to advance its fast paced pivotal vaccine trial.
Faster, Smarter, Better: How Analytics Are Boosting Clinical Data Quality Paragraph:
Data and analytics are being harnessed in really interesting, new ways to conduct smarter trials. Clinical trials are seeing an explosion of new data coming into the trial for analysis. So how are organizations keeping up with managing data quality on this kind of scale? This episode explores how the industry is boosting data quality with advanced analytics.