The Medidata Platform
臨床試験の一元管理
Medidata Platformは、患者、治験実施施設、スポンサー、パートナーを安全で拡張性の高いクラウド環境で結び、人生を変える治療を最も必要としている人々に提供します。
スプレッドシートは不要です。データ照合も不要です。複数のログインも不要です。1つの場所で、最初から最後まで試験を管理することができます。
これがメディデータの力です。
試験タイムラインを加速させる実証済みの方法
それは単なる話だけではありません。メディデータの顧客は、当社のソリューションとサービスを利用することで試験に実際に影響を及ぼし、記録的な速さで試験のマイルストーンに到達しています。
- PSを使用していないマッチング試験と比較したビルドタイム中央値の差の解析(p<0.05);30日の短縮
- EDC+少なくとも1つの追加製品 vs. EDCのみの試験におけるFPIからLPLVまでの期間中央値の差の解析(p<0.05)2017年~2021年;59日の短縮
- 2017年から2021年までのEDC+少なくとも1つの追加製品 vs. EDCのみの試験におけるLPLVからDBLまでの時間中央値の差の分析
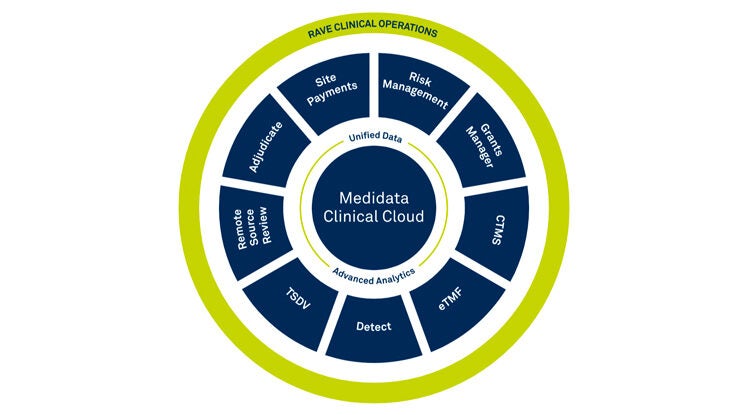
Medidata Platform Solutions
当社は業界唯一の臨床試験統合プラットフォームとして、ライフサイエンスおよび医療機器企業が開発コストを削減し、リスクを軽減し、治療法とデバイスをより早く市場に投入できるよう支援しています。
Rave、Patient Cloud、Medidata AIのいずれのソリューションを選択しても、Medidata Platformのパワーを利用することができます。
当社の強力なソリューションについては、以下をご覧ください。
患者さんのために、患者さんによって設計されたソリューション
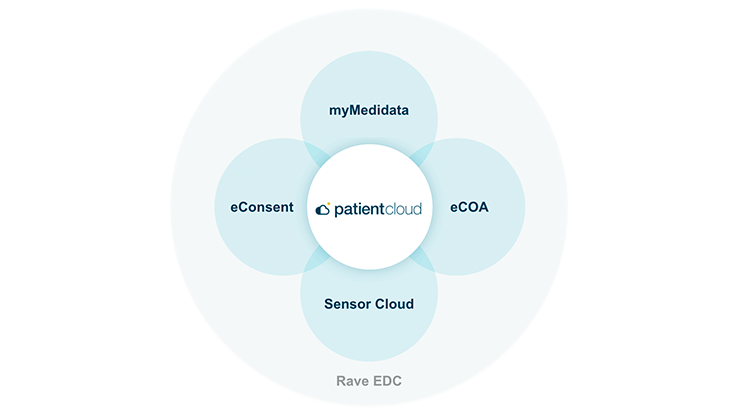
お客様のデータを、安全・安心・シームレスに管理するためのソリューション
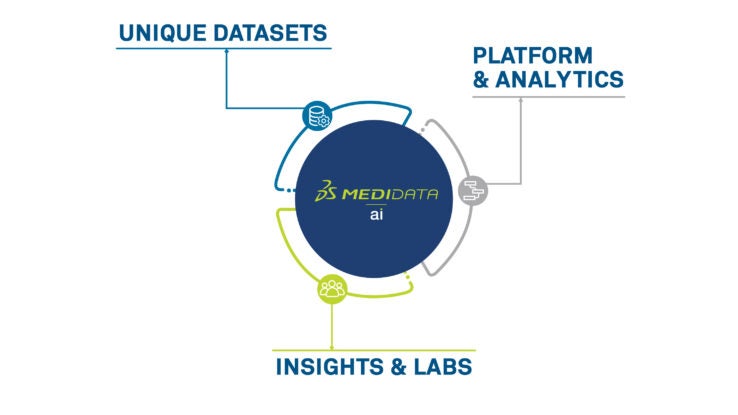
高度なアナリティクスを活用し、医薬品をより早く市場に投入するためのソリューション
1 Commercial Solutions
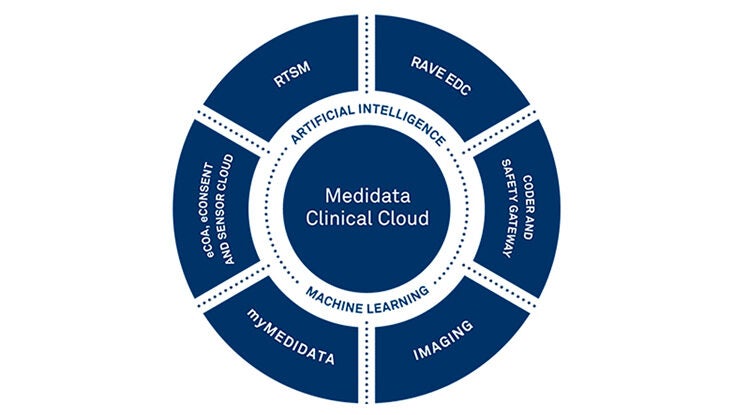
Medidata Platformの主な機能
Patient Experience
メディデータは、患者による患者のためのソリューションを提供する唯一の臨床試験テクノロジー・プロバイダーです。治験の前・中・後に患者を関与させ、当社の分散型臨床試験ソリューションの柔軟性を提供することで、当社はスポンサーがより多くの患者にとって臨床試験を選択肢にできるよう支援します。
当社の患者中心のソリューションは Rave EDC と統合され、Medidata Platform 上に構築されているため、追加の統合を必要とせずにデータの整合性を確保することができます。
Site Experience
弊社は、治験実施施設に管理業務ではなく、患者ケアに専念してもらいたいと考えています。これを念頭に置いて、弊社はMedidata Platformを治験実施施設にとってできるだけ使いやすいものにし、完了すべき重要なタスクを迅速に表示するとともに、データ入力をできるだけシームレスに行えるようにしました。
当社の分散型臨床試験ソリューションと組み合わせることで、治験施設は、クリニックと自宅のどちらにいても、患者のケアとモニタリングを柔軟に行える単一のプラットフォームを手に入れることができます。
Sponsor and Partner Experience
私たちは、次世代の臨床試験プラットフォームを構築しています。
高度な分析を用いて、各自の役割に合わせた単一のエクスペリエンスを構築し、ログインした瞬間に主要な洞察、タスク、データ、レポートが手元に届くようにしています。
CRA、データサイエンティスト、臨床業務管理者のいずれであっても、 Medidata Platformは業務を容易にするために構築されています。
詳細はこちら

中堅バイオ製薬企業のケーススタディ
COVID-19の流行時に、BioTissue社がどのように当社の統合プラットフォームを活用し、試験の要素を迅速に仮想化したかをご覧ください。

新興バイオテックの事例
Medidata Platformを利用して、希少疾病の患者と施設から選ばれるスポンサーになったRezoluteの事例をご覧ください。

新しいデータの未来への備え
メディデータは、新興企業から大手バイオ製薬企業まで、現在の臨床研究技術の意思決定者を対象に調査を行い、次世代の臨床研究に向けた技術インフラの準備状況について把握しました。