Rave eTMF
TMF작업, 보관, 관리에 최적화
Rave eTMF는 TMF 콘텐츠의 원활한 관리를 통해 시험에 맞춰 동시에 작성될 수 있도록 지원하는 뛰어난 보안을 자랑하는 글로벌 협업 플랫폼입니다.
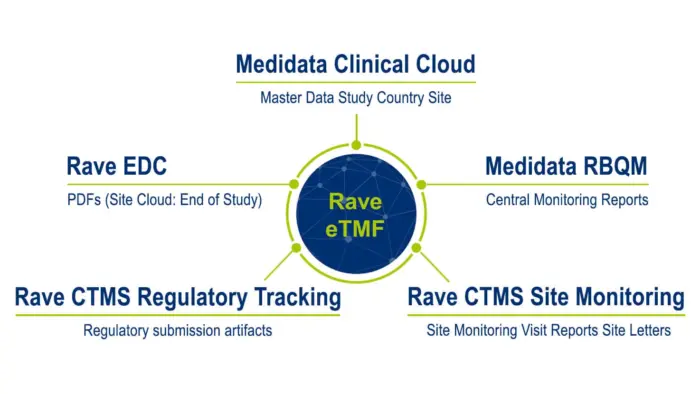
Rave eTMF는 다른 애플리케이션에서 생성 및 업데이트된 콘텐츠를 자동으로 입력함으로써 콘텐츠 생성 및 관리를 간소화합니다. 결과적으로 파일링 관련 수동 작업이 감소되며 단일 데이터 소스(Single Source of Truth)를 지원해 시험팀에게는 문서 생성 시간 및 시험기관 파일과 TMF 간 데이터 조정 시간 단축과 같은 상당한 효율성을 제공합니다. Rave eTMF는 또한 최소한의 IT 관여로 간단히 배포할 수 있어 실행 일정은 8주면 충분합니다.
제품 강점
임상시험 수행 간소화
Medidata Platform와의 호환을 바탕으로 콘텐츠가 Rave EDC 및 Rave CTMS로부터 자동으로 입력되어 TMF를 항상 완벽하게 유지할 수 있습니다. 그 결과 임상 시험 진행 상황에 대한 완벽한 파악을 지원하고, 실태조사 준비 상태를 보장하며, 시간과 리소스를 확보해 사용자가 가장 중요한 작업에 집중할 수 있도록 돕는 통합 솔루션이 탄생했습니다.
빠르고 정확하게 임상연구 진행
Rave eTMF의 계획 및 구현은 검증된 Agile 방법론을 사용합니다. 일반적으로 Rave eTMF 구성에서 Go-Live까지 8주면 가능합니다.
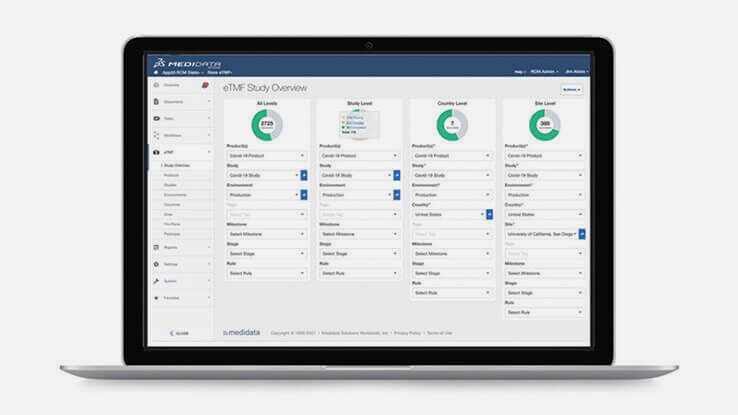
TMF 관리 간소화
Rave eTMF는 임상시험 전체 주기에서 콘텐츠와 데이터를 통합해 임상 문서 제출 프로세스를 간소화합니다. Medidata Platform의 일부인 Rave eTMF는 플랫폼 내 다른 애플리케이션의 콘텐츠와 데이터를 즉시 자동 입력할 수 있어 TMF는 항상 완성된 상태를 유지할 수 있습니다. 또한 새로운 임상시험 계획을 단 몇 분 내로 생성하고 파일 계획을 커스텀할 수 있습니다.
실시간 협업 강화
시험기관, 의뢰자, CRO 모두 직관적인 단일 애플리케이션에서 전체 TMF 주기에 대해 생성, 저장, 보기, 편집, 공동 작업을 할 수 있습니다.

주요 기능

종합적 검색
Rave eTMF의 고급 검색 알고리즘은 콘텐츠, 이름 및 메타데이터에 기반하여 TMF artifact 검색을 단순하고 정확하게 합니다. 자동 명명과 메타데이터를 이용하여 규제 및 비규제 콘텐츠를 모두 쉽게 검색하고 관리할 수 있는 표준화된 콘텐츠의 단일 플랫폼을 제공합니다.

TMF Reference Model
Rave eTMF는 DIA(Drug Information Association)의 TMF reference model에 대한 완벽한 지원을 제공하며, 즉시 사용 가능한 DIA 파일 계획 구성을 포함합니다.
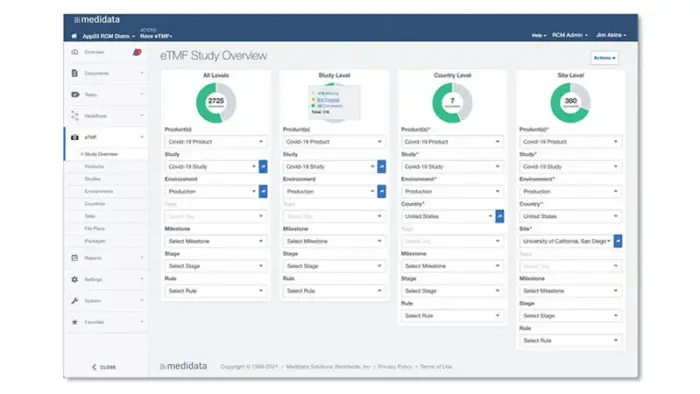
대시보드 및 리포팅
Rave eTMF는 포괄적인 대시보드 및 리포팅을 통해 제공되는 문서에 대한 실시간 감독 및 액세스를 바탕으로 사용자가 실태조사 준비 상태를 지속적으로 유지할 수 있도록 지원합니다.
관련 제품

Rave TSDV
Rave EDC와 호환되는 Rave TSDV (Targeted SDV)는 CRA가 위해성 관리 활동을 통해 확인된 품질결정(CtQ) 요소에 집중할 수 있도록 지원합니다. 또한 선택적 SDV 표적화를 진행할 Rave EDC 폴더, 양식 및 데이터 필드를 확인합니다.
추가 정보
통합 문서 관리를 통한 임상시험 감독 간소화
TMF의 복잡성 증가로 인해 하루에 수천 개에 달하는 문서에 대한 관리 및 엄격한 규제 제출 요건이 요구되고 있습니다. Rave eTMF는 협업 강화, 실시간 감독 및 자동화 문서 워크플로우를 통해 운영 효율성을 높입니다.

임상시험 모니터링 워크플로우 자동화를 통한 효율성 극대화
Enterin은 확인서 및 리포트 생성을 자동화하고 해당 데이터를 고위 경영진 및 시험기관 관리자와 보다 쉽게 공유하는 기능을 갖춘 솔루션을 위해 메디데이터의 도움을 구했습니다. Enterin은 Rave eTMF를 채택함으로써 매주 5~6시간을 절약하고 시험기관의 부담을 줄일 수 있었습니다.

Rave eTMF + Rave CTMS 제품 비디오
메디데이터 플랫폼에 통합된 Rave eTMF는 TMF 및 주요 artifact를 Rave CTMS에 자동으로 입력해 결과를 최적화하고 데이터 품질을 개선합니다. 또한 임상시험 일정을 가속화하고 위험을 줄임으로써 팀이 가치가 높은 활동에 집중할 수 있도록 합니다.