Your One Stop for All Your Drug and Clinical Development Needs
Whether you’re a startup, small, or mid-sized company, Medidata, a Dassault Systems company, offers more than Rave EDC. We provide the right-sized solution for each step of your clinical trial. Biotech and pharma organizations of all sizes rely on our technology to streamline and expedite their trials from protocol design to close-out across all phases (0-4). We’re your trusted partner throughout your clinical trial journey—whether you work with a CRO or not.
Explore our clinical trial technology roadmap to discover the breadth of technology we offer.
Our Customers Trust Us for Proven Results

Navigating Clinical Trial Complexities
“Medidata has made a tremendous impact on how MyMD Pharmaceuticals has been able to conduct its clinical research…Through the platform’s intuitive interface, our research teams can confidently navigate the complexities of the trial to ensure all data elements are captured efficiently. Medidata has also helped reduce the administrative burden of data collection, allowing the research teams to focus on patient outcomes.”
Jenna Brager PhD, RN
Executive VP, Drug Development

Highly Responsive Support
“The [Medidata] team, whether it’s on a technical level or a service level, has been very smooth, very responsive, and I always get help when we do an EDC build. They have input and suggestions on what we should and should not do to overcomplicate the study build.”
Salam Ammus
Executive Director, Clinical Data Management, Alkermes

Collaborative Partnerships
“Medidata has been a great partner to us. They like to hear what we have to say. We also collaborate and hear what Medidata has to say, and hope to come to a solution that works for Moderna.”
Margo Jaffee
VP of Clinical Trial Excellence and Support, Moderna

Built for Patient Engagement
“When we started working on the DCT [Decentralized Clinical Trial], we came to know about myMedidata from Medidata…It’s one of the really good tools that we implemented and our patients are happy they are getting enrolled. So, overall product suite-wise, Medidata is one-stop shopping ”
Mohit Goel
Associate Director of EDC Programming, Jazz Pharmaceuticals
One Technology Platform, One Experience for Patients, Sponsors, and Sites
Our platform connects Patients, Sites, and Sponsors providing a seamless user experience before, during, and after the trial for all patients, sites, sponsors and CROs.
Our proven innovative technology helps you adapt, simplify, and accelerate your clinical research across each step of your study. From protocol design to study startup, conduct, and close-out, we have the experience and expertise to help guide you through your clinical trial endeavors.
By infusing AI with automation to gain better insights with your trial data, you’ll mitigate risks, increase operational efficiencies, and improve trial outcomes.

Patient-centric Trials Demand a Patient-centric Approach
Clinical Data Studio’s AI-driven oversight gives a more accurate view of the patient—delivering faster, safer trials more efficiently.
Decrease Patient Burden to Avoid Recruitment and Retention Challenges
Medidata’s Patient Insights program infuses the patient perspective into the software development life cycle to improve the overall patient experience in clinical research operations. Medidata Patient Cloud’s commitment to patient centricity is unmatched by anyone else in the industry, as its technology is built for patients by patients.
See how our patient-centric clinical trials solutions help you support patients every step of the way, from enrollment to retention and beyond.
Make Data Entry Easier and Faster for Your Clinical Trial Sites
Rave Companion will reduce time spent on data entry, increase job satisfaction, reduce monitoring costs, and ultimately make for better, more efficient research.
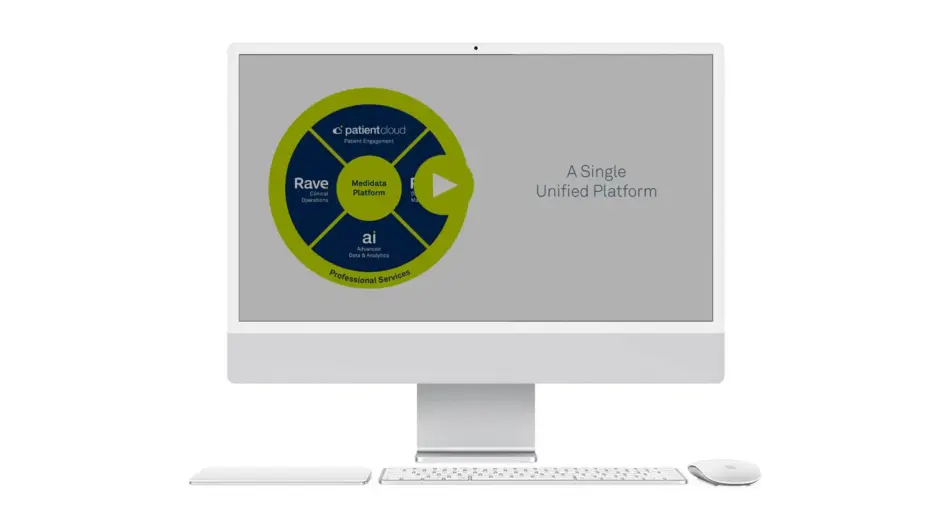
One Place to Manage Your Study from Start to Finish
Enhance your visibility across sites, drive operational efficiencies, and decrease trial timelines with automation using the Medidata Platform. It’s an intuitive cloud-based solution that works seamlessly with your existing systems and can support individual studies through large global programs.
See how our unified technology platform helps you streamline your trial processes so you can do more with less.
Meet Your Trial Diversity Goals
Lack of diversity in clinical trials has been a long-standing issue, and one with extensive implications. It can create gaps in the industry’s understanding of how treatments in disease areas affect minority participants and lead to unequitable access to novel treatments.
Build diversity into every step of your trial strategy and set your study up for success with data and analytics solutions that can help you elevate and future-proof your clinical trials.
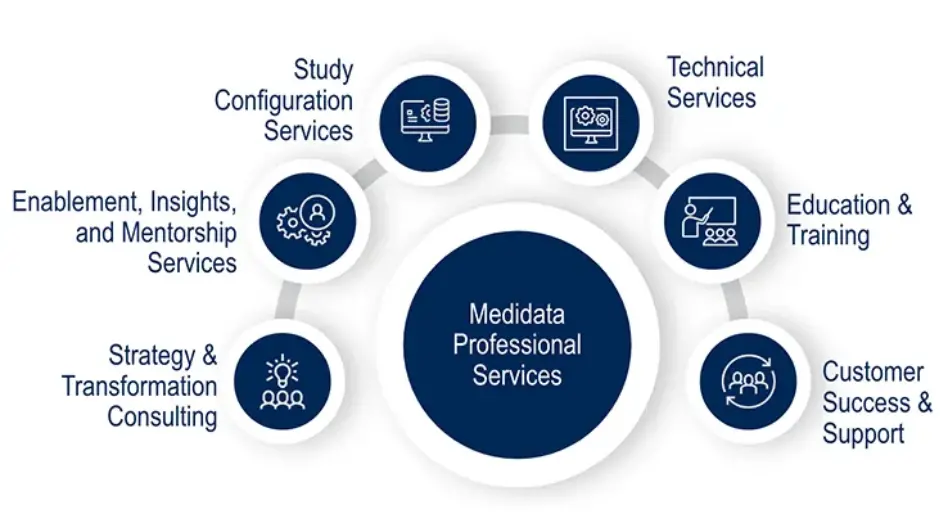
Realize Increased Value through Medidata Expertise and Innovation
Medidata has paved the way for the next generation of clinical studies including specialized solutions like decentralized clinical trials, patient-centric solutions, synthetic control arm studies, and advanced analytics.
We provide comprehensive professional services that support the entire clinical trial process, from pre-planning to post-go-live.
Proven to Transform Your Clinical Trial
It’s not just talk. Medidata customers are seeing real impact on their studies when using our solutions and services, reaching their study milestones in record time.
Reduce Your Study Builds by
ONE MONTH
When Using Medidata Professional Services
Conduct Studies
FIVE MONTHS
Faster When Using Multiple Products
Reach Database Lock
FOUR DAYS
Sooner When Using Multiple Products
- Analysis of difference in median build time vs. matched studies not using PS (p<0.05); Reduction of 30 days.
- Analysis of difference in median FPI to LPLV time for EDC + at least one additional product vs. EDC only studies (p<0.05) 2017 to 2021; Reduction of 59 days.
- Analysis of difference in median LPLV to DBL time for EDC + at least one additional product vs. EDC only studies from 2017 to 2021.
We Empower Biotech & Pharmaceutical Companies of All Sizes
For over 25 years we’ve been powering smarter treatments and healthier people through digital solutions to support the success of your clinical trials.
33,000+
Clinical Trials
Thousands of studies in 140+ countries have been conducted on the Medidata platform.
44K+
Sites
Tens of thousands of sites are using the Medidata’s decentralized clinical trial solutions and technology to conduct clinical trials today.
2,300+
Customers
Medidata studies, patients, and data are involved in nearly 40% of company-initiated trial starts globally.
10M+
Participants
Over 10 million patients across Medidata trials have impacted clinical research and touched countless lives.
Resources to Help Accelerate Your
Trial’s Success
Read the latest resources from our experts for insights on running successful clinical trials.
Ready to Transform Your Clinical Trials?
Talk to our experts to gain industry insights and guidance on your clinical trial journey and discover the right-sized solutions for your clinical trial needs.